Publications
For the most updated list, please visit my Google Scholar page.
2025
-
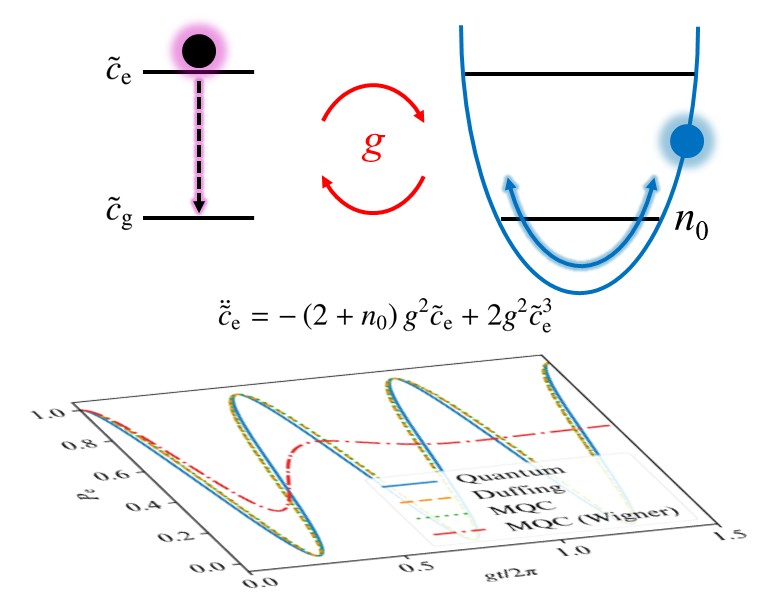 Mixed quantum–classical dynamics yields anharmonic Rabi oscillationsMing-Hsiu Hsieh and Roel TempelaarJ. Chem. Phys. 2025, 162, 224109
Mixed quantum–classical dynamics yields anharmonic Rabi oscillationsMing-Hsiu Hsieh and Roel TempelaarJ. Chem. Phys. 2025, 162, 224109This paper has been selsected as Cover and in the 2025 JCP Emerging Investigators Special Collection of the Journal of Chemical Physics, Volume 162, Issue 22, June 2025.
We apply a mixed quantum–classical (MQC) approach to the quantum Rabi model, involving a classical optical field coupled self-consistently to a quantum two-level system. Under the rotating wave approximation, we analytically show that this approach yields persistent yet anharmonic Rabi oscillations, governed by an undamped and unforced Duffing equation. We consider the single-quantum limit, where we find that such anharmonic Rabi oscillations closely follow full-quantum results once zero-point energy is approximately enforced when initializing the optical field coordinate. Our findings provide guidance in the application of MQC dynamics to classes of problems involving small quantum numbers and far away from decoherence.
-
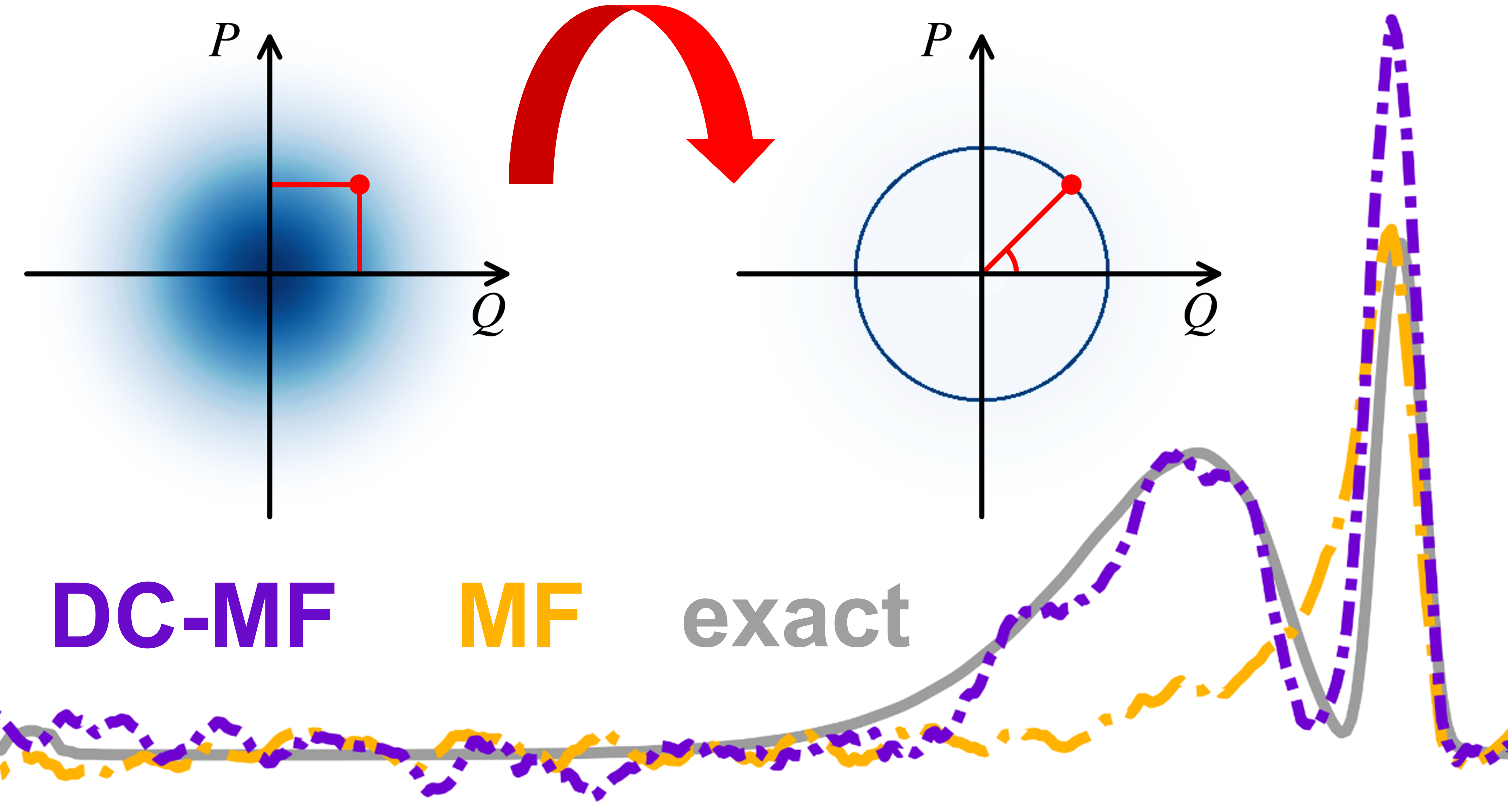 Focused Sampling for Low-Cost and Accurate Ehrenfest Modeling of Cavity Quantum ElectrodynamicsMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Chem. Theory Comput. 2025, 21, 11860–11868
Focused Sampling for Low-Cost and Accurate Ehrenfest Modeling of Cavity Quantum ElectrodynamicsMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Chem. Theory Comput. 2025, 21, 11860–11868An economic modeling approach for cavity quantum electrodynamics is provided by mean-field dynamics, wherein the optical field is described classically while a self-consistent interaction with quantum emitters is incorporated through the Ehrenfest theorem. However, conventional implementations of mean-field dynamics are known to suffer from a catastrophic leakage of zero-point energy, to lose accuracy in the short-cavity limit, and to require large numbers of trajectories to be sampled. Here, we address these three shortcomings within a single integrated approach. This approach builds on our recently-proposed modification of the Ehrenfest theorem, referred to as decoupled mean-field (DC-MF) dynamics, in combination with a focused sampling scheme that enforces zero-point energy at the single-trajectory level. The approach is shown to yield high accuracy in both short and long-cavity limits while reaching convergence within a minimal amount of trajectories.
2023
-
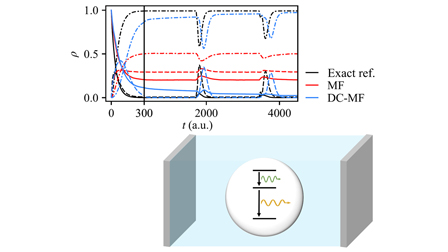 Ehrenfest Modeling of Cavity Vacuum Fluctuations and How to Achieve Emission from a Three-Level AtomMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Chem. Phys. 2023, 159, 221104
Ehrenfest Modeling of Cavity Vacuum Fluctuations and How to Achieve Emission from a Three-Level AtomMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Chem. Phys. 2023, 159, 221104This paper has been selsected as a Rapid Communcation for “highly significant work whose rapid publication will be important to a relatively large number of workers in the field.”
A much-needed solution for the efficient modeling of strong coupling between matter and optical cavity modes is offered by mean-field mixed quantum–classical dynamics, where a classical cavity field interacts self-consistently with quantum states of matter through Ehrenfest’s theorem. We previously introduced a modified mean-field approach, referred to as decoupled mean-field (DC-MF) dynamics, wherein vacuum fluctuations of the cavity field are decoupled from the quantum-mechanical ground state as a means to resolve an unphysical drawing of energy from the vacuum fluctuations by a two-level atom. Here, we generalize DC-MF dynamics for an arbitrary number of (nondegenerate) atomic levels and show that it resolves an unphysical lack of emission from a three-level atom predicted by conventional mean-field dynamics. We furthermore show DC-MF to provide an improved description of reabsorption and (resonant) two-photon emission processes.
-
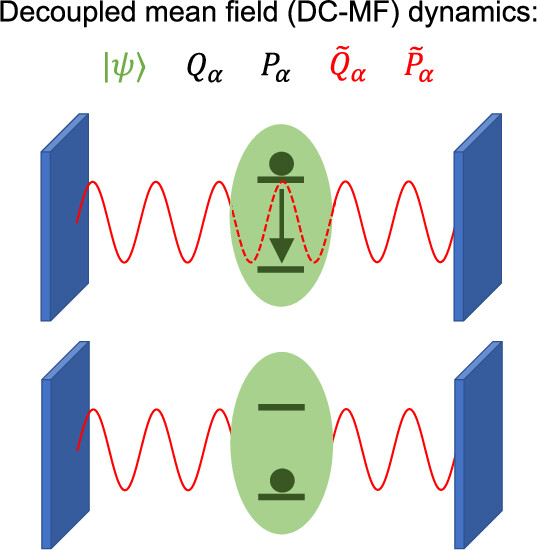 A Mean-Field Treatment of Vacuum Fluctuations in Strong Light–Matter CouplingMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Phys. Chem. Lett. 2023, 14, 1253–1258
A Mean-Field Treatment of Vacuum Fluctuations in Strong Light–Matter CouplingMing-Hsiu Hsieh, Alex Krotz, and Roel TempelaarJ. Phys. Chem. Lett. 2023, 14, 1253–1258Mean-field mixed quantum–classical dynamics could provide a much-needed means to inexpensively model quantum electrodynamical phenomena by describing the optical field and its vacuum fluctuations classically. However, this approach is known to suffer from an unphysical transfer of energy out of the vacuum fluctuations when the light–matter coupling becomes strong. We highlight this issue for the case of an atom in an optical cavity and resolve it by introducing an additional set of classical coordinates to specifically represent vacuum fluctuations whose light–matter interaction is scaled by the instantaneous ground-state population of the atom. This not only rigorously prevents the aforementioned unphysical energy transfer but is also shown to yield a radically improved accuracy in terms of the atomic population and the optical field dynamics, generating results in excellent agreement with full quantum calculations. As such, the resulting method emerges as an attractive solution for the affordable modeling of strong light–matter coupling phenomena involving macroscopic numbers of optical modes.
2022
-
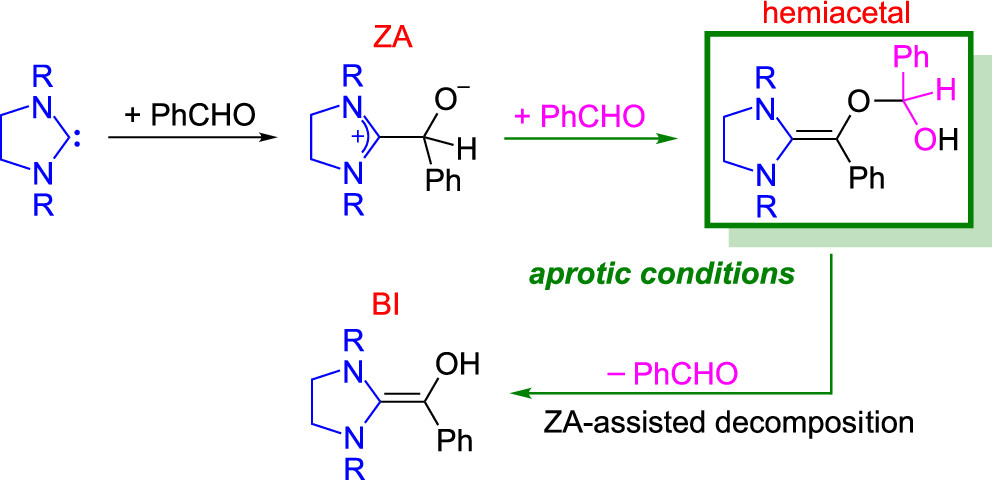 Formation of Breslow Intermediates under Aprotic Conditions: A Computational StudyGou-Tao Huang, Ming-Hsiu Hsieh, and Jen-Shiang K. YuJ. Org. Chem. 2022, 87, 2501–2507
Formation of Breslow Intermediates under Aprotic Conditions: A Computational StudyGou-Tao Huang, Ming-Hsiu Hsieh, and Jen-Shiang K. YuJ. Org. Chem. 2022, 87, 2501–2507The mechanism of formation of the Breslow intermediate (BI) under aprotic conditions is investigated with density functional theory (DFT) calculations. The zwitterionic adduct (ZA) is formed by the first addition of an imidazolinylidene to benzaldehyde. The forward reaction is found to proceed through the second addition of the ZA to another benzaldehyde, and subsequent proton migration gives a hemiacetal. The bimolecular reaction enables the conversion of the ZA to a more reactive hemiacetal, which is further decomposed to the BI with the assistance of the ZA. During the ZA-assisted process, the hemiacetal and the BI act as hydrogen bond donors to stabilize the ZA. The hydrogen bond interactions between the ZA and the BI or hemiacetal are analyzed. The DFT computations demonstrate that along the proposed route, the proton migration leading to the hemiacetal intermediate is the rate-determining step (∆G⧧ = 21.2 kcal mol–1). The bimolecular mechanism provides an alternative pathway to explain BI formation under aprotic conditions.
2021
-
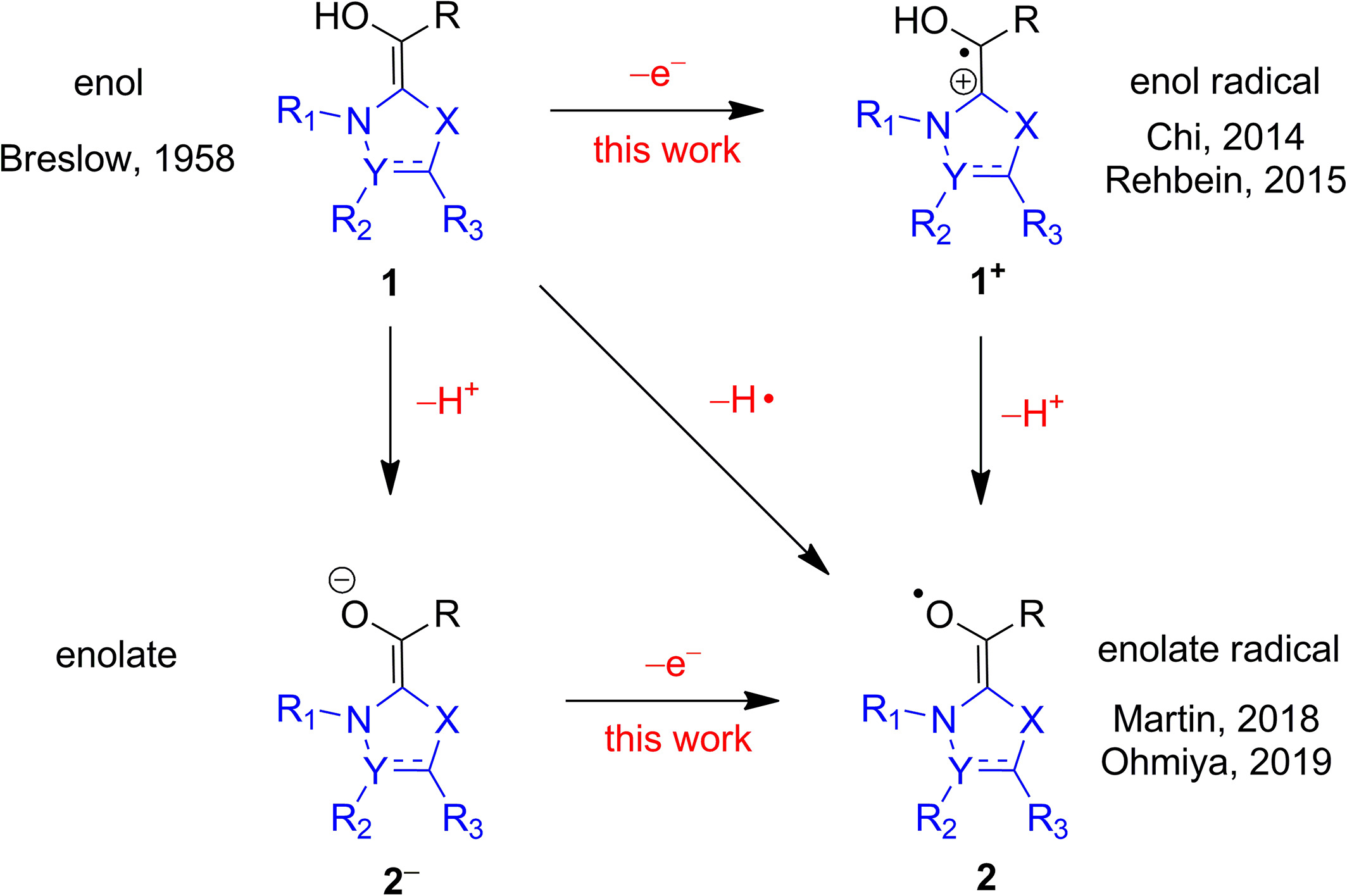 Dipole-Bound States and Substituent Effects of Breslow Intermediates in the Enolate FormMing-Hsiu Hsieh, Gou-Tao Huang, and Jen-Shiang K. YuJ. Chin. Chem. Soc. 2021, 68, 2060–2070
Dipole-Bound States and Substituent Effects of Breslow Intermediates in the Enolate FormMing-Hsiu Hsieh, Gou-Tao Huang, and Jen-Shiang K. YuJ. Chin. Chem. Soc. 2021, 68, 2060–2070Breslow intermediates play crucial roles in both umpolung and redox reactions in N-heterocyclic carbene catalysis. Compared to the well-known nucleophilic character, the electronic structure of Breslow intermediates on the radical route is still unclear. We investigate the potential energy surfaces with high-level ab initio methods for four typical Breslow intermediates in both of their enol and enolate forms. In the enol form, high energies of around 60 kcal/mol to the Rydberg-like states and those higher than 120 kcal/mol to remove an electron demonstrate that the enol Breslow intermediates tend not to generate radicals unless strong oxidants are present. The low-lying dipole-bound states and small electron detachment energies in the enolate form in contrast show that the enolate Breslow intermediates are possible precursors to radicals. More importantly, metastable dipole-bound states exist in the imidazole- and the triazole-based enolate Breslow intermediates. Energies to detach one electron of several enolate Breslow intermediates reveal that the bulky and electron-withdrawing groups stabilize the singlet ground states, which explains that the utilization of such substituents can lead to successful isolation for Breslow intermediates in experiments.
-
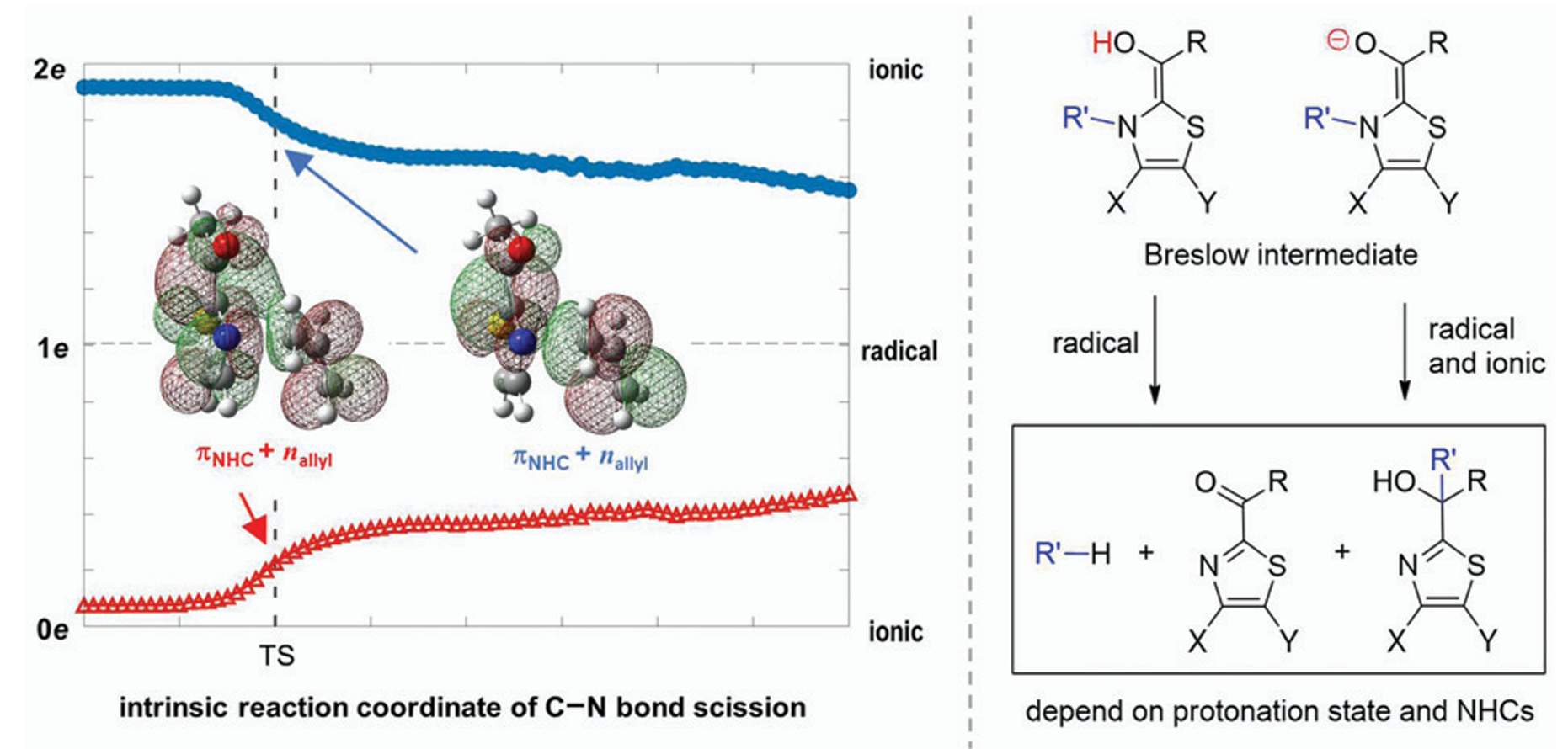 Fragmentation and Rearrangement of Breslow Intermediates: Branches to Both Radical and Ionic PathwaysMing-Hsiu Hsieh and Jen-Shiang K. YuPhys. Chem. Chem. Phys. 2021, 23, 27377–27384
Fragmentation and Rearrangement of Breslow Intermediates: Branches to Both Radical and Ionic PathwaysMing-Hsiu Hsieh and Jen-Shiang K. YuPhys. Chem. Chem. Phys. 2021, 23, 27377–27384Breslow intermediates are the key species in N-heterocyclic carbene-catalyzed reactions to promote the C–C bond formation. As the fragmentation and rearrangement of Breslow intermediates terminate the catalytic cycle of N-heterocyclic carbene, two mechanisms under debate have been proposed in terms of the radical channel and the ionic route. Theoretical calculations demonstrate herein that ionic and radical characteristics can coexist, depending on the protonation state of the hydroxyl group in Breslow intermediates: radicals are merely generated in the enol system, while both ionic and radical species exist in the enolate system with a lower barrier. Complete pathways for thiamin analogue and N-allyl benzothiazole Breslow intermediates are exclusively constructed considering experimental conditions. The growing population of the enolate under higher pH values rationalizes the increased rate of the fragmentation of thiamin. The fragmentation products of thiamin, namely pyrimidine and ketone, are the thermodynamic products, while the tertiary alcohol is both the kinetic and thermodynamic product for N-allyl benzothiazole Breslow intermediate via a Claisen-like rearrangement. Other NHCs used to synthesize tertiary alcohols could form the enolate due to the base, followed by the production of stable radicals and recombination to form tertiary alcohols. It is concluded that specific protonation states and chemical structures of NHCs account for the distinct mechanisms.
2018
-
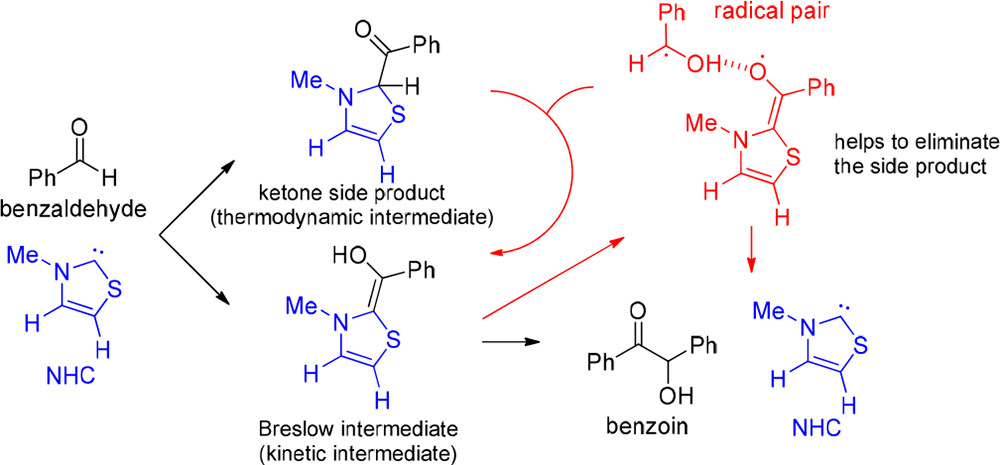 Can the Radical Channel Contribute to the Catalytic Cycle of N-Heterocyclic Carbene in Benzoin Condensation?Ming-Hsiu Hsieh, Gou-Tao Huang, and Jen-Shiang K. YuJ. Org. Chem. 2018, 83, 15202–15209
Can the Radical Channel Contribute to the Catalytic Cycle of N-Heterocyclic Carbene in Benzoin Condensation?Ming-Hsiu Hsieh, Gou-Tao Huang, and Jen-Shiang K. YuJ. Org. Chem. 2018, 83, 15202–15209NHC can catalyze benzoin condensation via the key Breslow intermediate. EPR spectroscopy recently confirmed the existence of the radical species, but its catalytic role is still unclear. Herein, we use density functional approaches to study the radical-associated pathway in comparison with the nonradical mechanism reported previously. Theoretical investigations show that the nonradical path (∆G⧧ = 18.7 kcal/mol) is more kinetically favorable than the radical route (∆G⧧ = 27.6 kcal/mol), which is initialized by the hydrogen abstraction from the Breslow intermediate by benzaldehyde, leading to a radical pair. The product formation is thus dominated by the nonradical pathway. In addition, the Breslow intermediate is less stable than its keto form, which blocks the benzoin condensation, and the radical species could play an important role in assisting the tautomerization and promoting the catalytic reaction.